Research Ethics
This page contains information to assist faculty and students in obtaining approvals to conduct research that involves humans, animals, and biohazards. All Ethics Application Forms (Human, Animal, and Biohazard) are accessible through the Romeo software system. Please click here for more information on Romeo.
Wondering where to start?
See our Ethics FAQ
Human research ethics
Read the Research Ethics Board Terms of Reference (REB ToR).
The Review Process and Approximate Timelines for Minimal Risk Research
The REB’s goal is to provide a review to researchers within four weeks. This feedback will be sent to you through ROMEO. It will include instructions on how to revise and resubmit your application form based on the review/feedback. After you have re-submitted your application, if items are still outstanding, then the REB will request further revisions and clarifications. If no further clarification or revisions are required, then the REB will send you a Letter of Approval, and you can begin contacting human participants for data collection.
The time to obtain certification depends on several factors including volume of REB applications under review at the time. Reviews tend to take a little longer during peak periods such as September/October, and during vacation seasons such as August and over Christmas. Please keep this in mind as you plan your research project.
Instructions for submitting a new REB application form in ROMEO
How do I submit an REB application in ROMEO?
Checklist of materials to include with your application
- Informed consent form. Please use this checklist to ensure that your consent form includes all the necessary information. Incomplete consent forms will be returned to you for completion.
- TCPS 2 Certificate of Completion
- Recruitment scripts such as: email script, poster, social media post, telephone script … word-for-word what you will say when you initiate contact with potential participants.
- Data collection tools such as: survey, questionnaire, interview questions, focus group discussion guide.
- Official letters of support to work with communities and organizations such as Indigenous communities and school districts. Please note that official letters of support come on letters with the organization’s letterhead. Email would not suffice.
- It may be appropriate for you to obtain a confidentiality agreement such as from your research assistants or from participants of focus groups, transcriptionists, and other circumstances. Please revise the following as needed for your study:
- Supervisor declaration. All student research must be supervised by a TRU faculty member. Supervisors must sign a declaration that states they have read and approve the submission of their student's application for review by the REB. REB review of student research will begin only after this declaration is attached to the romeo file.
- Instructions for submitting a new REB application in ROMEO
Instructions for submitting and retrieving amendments and renewals in ROMEO
How to submit and retrieve amendments and renewal documents in your approved file in Romeo?
Please note that only Principal Investigators (PI) can submit applications. Only the PI has the submit button on their screen.
- Log into your account
- Search for the study you want to amend
- You can enter the REB ROMEO file number in the search bar
- Or search for it in your list of files (often it is in Applications: Post Review)
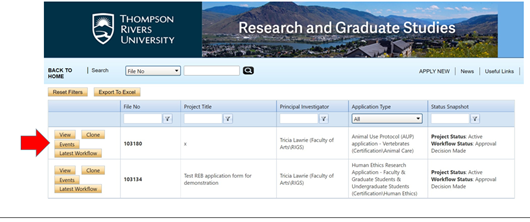
- Open the file and click on “events”
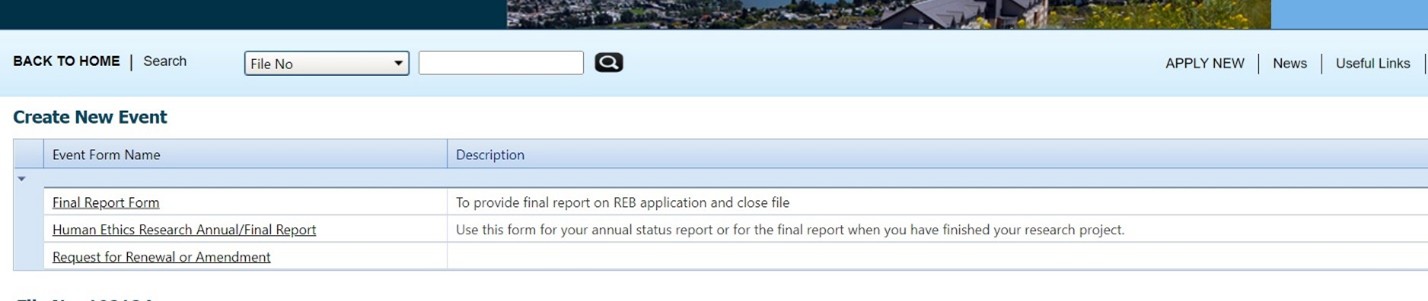
- Choose “request for renewal or amendment”
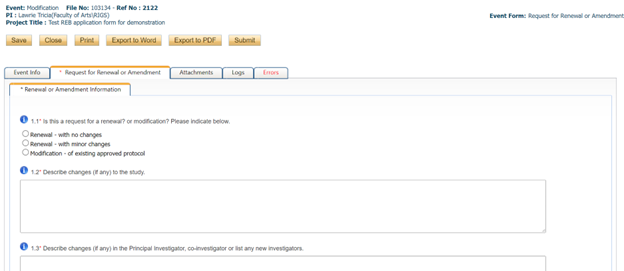
- Answer all of the questions in the “request for renewal or amendment” tab
- Submit any additional documents in the attachments tab (TCPS2 certificates, revised consent forms, revised data collection tools or surveys, etc.)
- The “errors” tab will inform you of any mandatory questions/fields that you missed. You will not be able to submit until all errors are addressed.
- Click “SUBMIT” please note that only the Principal Investigator will have a submit button. This is because the PI is ultimately accountable for the ethical conduct during the project and thus should oversee and approve all submitted materials and information
- ROMEO will require that you comment before you can submit. Just a brief phrase or a sentence or two.
Provided the committee has all the required information, approval should take about a week depending on current volume.
***Please note that (especially in the case of amendments) the committee may have further questions or comments just to ensure that your study protocol is in compliance with the Tri-Council, the Canadian Council for Animal Care, or the Public Health Agency of Canada as the case may be. It is always a good plan to apply early in anticipation of feedback from the committees before approval is granted.
Animal Care Committee
Submission Deadline: Continuous Intake
Thompson Rivers University (TRU) regards the use of animals in research, teaching, testing and production as a privilege and not a right. All individuals involved in the use of animals for research, teaching, testing and production must care for their animals in compliance with the TRU Animal Care Committee (TRU-ACC) protocols, the Canadian Council for Animal Care (CCAC) Guidelines and in accordance with Sections 444-447 of the Criminal Code of Canada and Section 24 of the Prevention of Cruelty to Animals Act.
Thompson Rivers University treats all animals used in teaching and research with the highest standards of ethical care and treatment, and has been recognized with a Certificate of Good Animal Practice issued by the Canadian Council on Animal Care.
Biosafety Committee
All Biosafety resources are now found in the TRU Romeo system. This includes Biosafety Application and Standard Operating Procedure forms.
Please refer to our Biosafety website for additional information. https://www.tru.ca/hsafety/biosafety.html
This office is supported by the federal Research Support Fund. The Fund assists Canadian post-secondary institutions to maintain a world-class research environment, which includes human ethics, animal care and biosafety.
